Oxidation Reduction Titration Lab Report Discussion . It is one of the most common laboratory. Redox titration includes oxidation half. this is a redox titration. oxidation is a loss of electrons. At the end of a chemical reaction, the net gain of electrons must be equal to the loss of electrons. Therefore, oxidation results in an increase in oxidation. I 2 + 2e ⎯ → 2i ⎯. Reduction half reaction for iodine at ph 5: redox titration is the type of titration based on redox reaction between the analyte and titrant. oxidation can be defined as loss of electrons and reduction as gain of electrons. The two relevant half reactions for reaction 10.2 above are:
from www.numerade.com
redox titration is the type of titration based on redox reaction between the analyte and titrant. At the end of a chemical reaction, the net gain of electrons must be equal to the loss of electrons. The two relevant half reactions for reaction 10.2 above are: oxidation can be defined as loss of electrons and reduction as gain of electrons. oxidation is a loss of electrons. I 2 + 2e ⎯ → 2i ⎯. It is one of the most common laboratory. this is a redox titration. Therefore, oxidation results in an increase in oxidation. Reduction half reaction for iodine at ph 5:
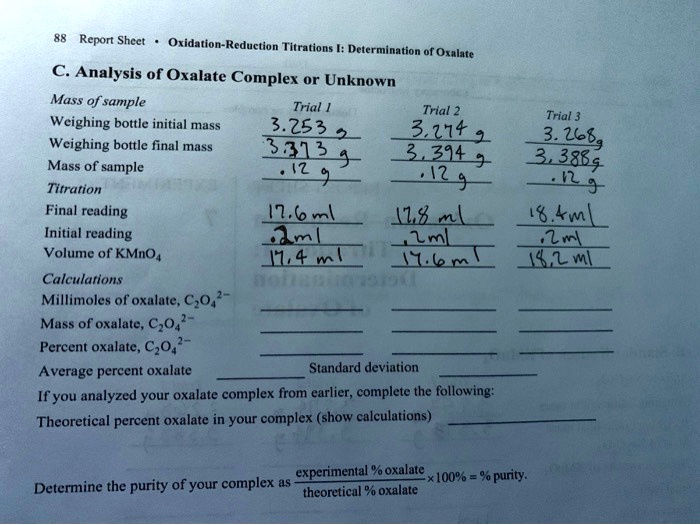
SOLVED Text Report Sheet OxidationReduction Titrations Determination of Oxalate C Analysis of
Oxidation Reduction Titration Lab Report Discussion redox titration is the type of titration based on redox reaction between the analyte and titrant. Therefore, oxidation results in an increase in oxidation. oxidation can be defined as loss of electrons and reduction as gain of electrons. I 2 + 2e ⎯ → 2i ⎯. redox titration is the type of titration based on redox reaction between the analyte and titrant. At the end of a chemical reaction, the net gain of electrons must be equal to the loss of electrons. this is a redox titration. Reduction half reaction for iodine at ph 5: It is one of the most common laboratory. Redox titration includes oxidation half. The two relevant half reactions for reaction 10.2 above are: oxidation is a loss of electrons.
From www.chegg.com
Solved EXPERIMENT 7 OXIDATIONREDUCTION TITRATIONS 4. Oxidation Reduction Titration Lab Report Discussion Redox titration includes oxidation half. At the end of a chemical reaction, the net gain of electrons must be equal to the loss of electrons. The two relevant half reactions for reaction 10.2 above are: redox titration is the type of titration based on redox reaction between the analyte and titrant. oxidation can be defined as loss of. Oxidation Reduction Titration Lab Report Discussion.
From www.chegg.com
OxidationReduction Titration Part LStandardization Oxidation Reduction Titration Lab Report Discussion The two relevant half reactions for reaction 10.2 above are: Reduction half reaction for iodine at ph 5: oxidation can be defined as loss of electrons and reduction as gain of electrons. At the end of a chemical reaction, the net gain of electrons must be equal to the loss of electrons. this is a redox titration. . Oxidation Reduction Titration Lab Report Discussion.
From www.chegg.com
Solved EXPERIMENT 7 OXIDATIONREDUCTION TITRATIONS 4. Oxidation Reduction Titration Lab Report Discussion Redox titration includes oxidation half. I 2 + 2e ⎯ → 2i ⎯. Therefore, oxidation results in an increase in oxidation. It is one of the most common laboratory. oxidation can be defined as loss of electrons and reduction as gain of electrons. this is a redox titration. oxidation is a loss of electrons. At the end. Oxidation Reduction Titration Lab Report Discussion.
From www.studocu.com
RT lab report Redox Titration Redox Titration Abstract The process of redox analysis is Oxidation Reduction Titration Lab Report Discussion oxidation is a loss of electrons. At the end of a chemical reaction, the net gain of electrons must be equal to the loss of electrons. redox titration is the type of titration based on redox reaction between the analyte and titrant. Therefore, oxidation results in an increase in oxidation. I 2 + 2e ⎯ → 2i ⎯.. Oxidation Reduction Titration Lab Report Discussion.
From cezisuit.blob.core.windows.net
Redox Titration Using Sodium Thiosulphate Lab Report Discussion at Lee Palumbo blog Oxidation Reduction Titration Lab Report Discussion this is a redox titration. I 2 + 2e ⎯ → 2i ⎯. Therefore, oxidation results in an increase in oxidation. oxidation is a loss of electrons. The two relevant half reactions for reaction 10.2 above are: redox titration is the type of titration based on redox reaction between the analyte and titrant. Reduction half reaction for. Oxidation Reduction Titration Lab Report Discussion.
From studylib.net
An OxidationReduction Titration The Reaction of Fe and Ce INTRODUCTION Oxidation Reduction Titration Lab Report Discussion Therefore, oxidation results in an increase in oxidation. At the end of a chemical reaction, the net gain of electrons must be equal to the loss of electrons. oxidation can be defined as loss of electrons and reduction as gain of electrons. It is one of the most common laboratory. The two relevant half reactions for reaction 10.2 above. Oxidation Reduction Titration Lab Report Discussion.
From www.vernier.com
OxidationReduction Titrations > Experiment 19 from Investigating Chemistry through Inquiry Oxidation Reduction Titration Lab Report Discussion The two relevant half reactions for reaction 10.2 above are: I 2 + 2e ⎯ → 2i ⎯. redox titration is the type of titration based on redox reaction between the analyte and titrant. oxidation is a loss of electrons. Reduction half reaction for iodine at ph 5: At the end of a chemical reaction, the net gain. Oxidation Reduction Titration Lab Report Discussion.
From www.studypool.com
SOLUTION Analytical chemistry lab oxidation reduction titration Studypool Oxidation Reduction Titration Lab Report Discussion oxidation is a loss of electrons. Therefore, oxidation results in an increase in oxidation. this is a redox titration. Reduction half reaction for iodine at ph 5: oxidation can be defined as loss of electrons and reduction as gain of electrons. redox titration is the type of titration based on redox reaction between the analyte and. Oxidation Reduction Titration Lab Report Discussion.
From www.vrogue.co
Redox Titration Lab Sheet 2 0 Final Name Date vrogue.co Oxidation Reduction Titration Lab Report Discussion oxidation is a loss of electrons. I 2 + 2e ⎯ → 2i ⎯. redox titration is the type of titration based on redox reaction between the analyte and titrant. Therefore, oxidation results in an increase in oxidation. Redox titration includes oxidation half. The two relevant half reactions for reaction 10.2 above are: this is a redox. Oxidation Reduction Titration Lab Report Discussion.
From partokarnokinos.blogspot.com
redox titration lab report im at a point in my life Oxidation Reduction Titration Lab Report Discussion this is a redox titration. The two relevant half reactions for reaction 10.2 above are: At the end of a chemical reaction, the net gain of electrons must be equal to the loss of electrons. It is one of the most common laboratory. oxidation is a loss of electrons. Redox titration includes oxidation half. redox titration is. Oxidation Reduction Titration Lab Report Discussion.
From www.studypool.com
SOLUTION Analytical chemistry lab oxidation reduction titration Studypool Oxidation Reduction Titration Lab Report Discussion The two relevant half reactions for reaction 10.2 above are: Therefore, oxidation results in an increase in oxidation. I 2 + 2e ⎯ → 2i ⎯. oxidation can be defined as loss of electrons and reduction as gain of electrons. this is a redox titration. Reduction half reaction for iodine at ph 5: At the end of a. Oxidation Reduction Titration Lab Report Discussion.
From www.chegg.com
Solved REPORT SHEET OxidationReduction Titrations II Oxidation Reduction Titration Lab Report Discussion this is a redox titration. It is one of the most common laboratory. The two relevant half reactions for reaction 10.2 above are: Therefore, oxidation results in an increase in oxidation. I 2 + 2e ⎯ → 2i ⎯. Reduction half reaction for iodine at ph 5: oxidation can be defined as loss of electrons and reduction as. Oxidation Reduction Titration Lab Report Discussion.
From www.studypool.com
SOLUTION Analytical chemistry lab oxidation reduction titration Studypool Oxidation Reduction Titration Lab Report Discussion redox titration is the type of titration based on redox reaction between the analyte and titrant. The two relevant half reactions for reaction 10.2 above are: this is a redox titration. At the end of a chemical reaction, the net gain of electrons must be equal to the loss of electrons. Redox titration includes oxidation half. oxidation. Oxidation Reduction Titration Lab Report Discussion.
From studylib.net
Redox Titration Oxidation Reduction Titration Lab Report Discussion this is a redox titration. It is one of the most common laboratory. Redox titration includes oxidation half. The two relevant half reactions for reaction 10.2 above are: At the end of a chemical reaction, the net gain of electrons must be equal to the loss of electrons. oxidation can be defined as loss of electrons and reduction. Oxidation Reduction Titration Lab Report Discussion.
From www.scribd.com
Experiment 7 Oxidation Reduction Titrations PDF Titration Chemistry Oxidation Reduction Titration Lab Report Discussion Reduction half reaction for iodine at ph 5: At the end of a chemical reaction, the net gain of electrons must be equal to the loss of electrons. oxidation is a loss of electrons. this is a redox titration. Therefore, oxidation results in an increase in oxidation. oxidation can be defined as loss of electrons and reduction. Oxidation Reduction Titration Lab Report Discussion.
From www.chegg.com
Solved Experiment 23/OxidationReduction Titration 2017 Name Oxidation Reduction Titration Lab Report Discussion At the end of a chemical reaction, the net gain of electrons must be equal to the loss of electrons. oxidation is a loss of electrons. Reduction half reaction for iodine at ph 5: I 2 + 2e ⎯ → 2i ⎯. It is one of the most common laboratory. redox titration is the type of titration based. Oxidation Reduction Titration Lab Report Discussion.
From www.chegg.com
Solved 230 Report Sheet OxidationReduction Titrations II Oxidation Reduction Titration Lab Report Discussion It is one of the most common laboratory. oxidation is a loss of electrons. Therefore, oxidation results in an increase in oxidation. The two relevant half reactions for reaction 10.2 above are: At the end of a chemical reaction, the net gain of electrons must be equal to the loss of electrons. this is a redox titration. I. Oxidation Reduction Titration Lab Report Discussion.
From www.scribd.com
ReductionOxidation Titration 2 PDF Titration Chemistry Oxidation Reduction Titration Lab Report Discussion Therefore, oxidation results in an increase in oxidation. Redox titration includes oxidation half. At the end of a chemical reaction, the net gain of electrons must be equal to the loss of electrons. this is a redox titration. It is one of the most common laboratory. oxidation can be defined as loss of electrons and reduction as gain. Oxidation Reduction Titration Lab Report Discussion.